Tolerance and Inflammation in Immunity
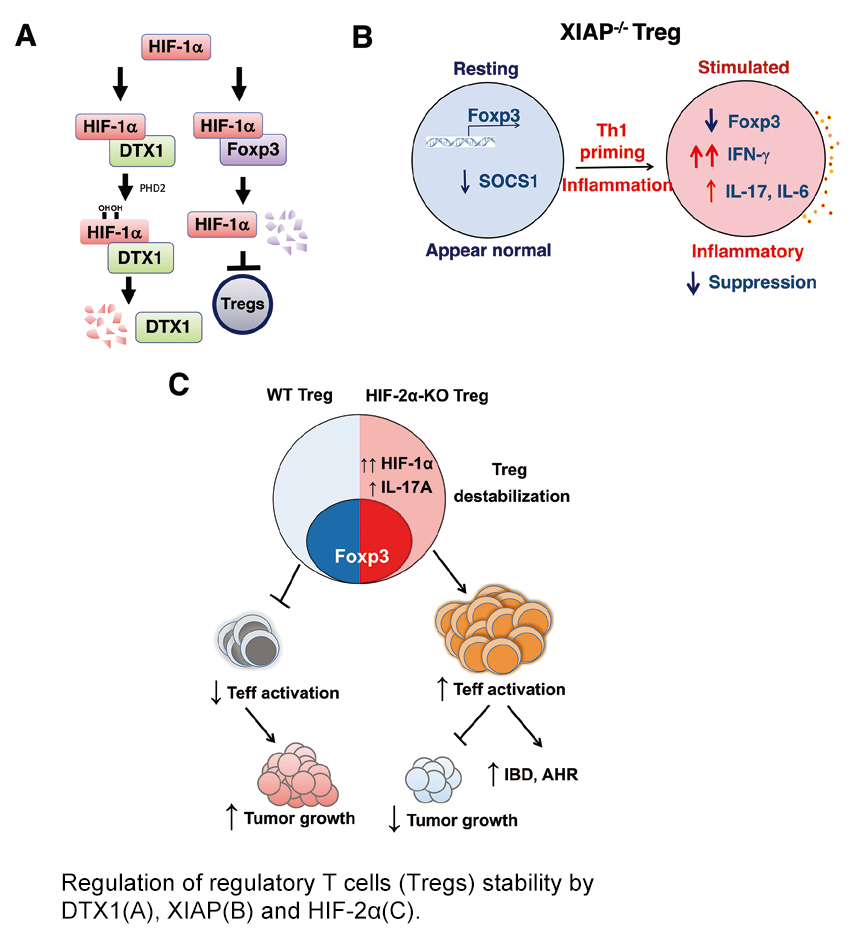
The primary interests of our lab are on the immune cell signaling and the balance between T cell tolerance and inflammation. We identified a critical role of deltex1 (DTX1) in T cell tolerance. Dtx1 is a transcription target of NFAT and is up-regulated in T cell anergy. Deficiency of DTX1 augments T cell activation, confers resistance to anergy induction, enhances autoantibody generation, and increases inflammation. Regulatory T cells (Tregs) suppress excess immune cells activation to maintain immune tolerance. We identified a specific role for DTX1 in the maintenance of Foxp3 protein stability and Treg inhibitory activity in vivo; DTX1 protects Foxp3 from HIF-1α-mediated downregulation in inflammatory tissues (A). We also identified another tolerance-associated molecule, death associated protein kinase (DAPK). Deficiency in DAPK leads to preferential differentiation of Th17 cells and development of experimental autoimmune encephalomyelitis. Th17 differentiation is accompanied by DAPK down-regulation and HIF-1α upregulation. In addition, X-linked inhibitor of apoptosis protein (XIAP) is essential in maintaining Tregs stability. Xiap-/- Tregs are prone to IFN-γ secretion and are defective in suppressive function. Xiap-/- Tregs display diminished SOCS1 expression, essential for suppressing inflammatory cytokine signaling (B). The impaired function of Tregs contributes to X-linked lymphoproliferative syndrome type-2. We further identified an unexpected role of HIF-2α for Tregs function. Despite normal development, HIF-2α-KO Tregs lose their in vivo suppressive functions, and are susceptible to reprogramming into IL-17-secreting inflammatory cells. Mice with Treg-conditional KO of HIF-2α are sensitive to inflammatory disease development, yet are resistant to carcinogenesis, illustrating the indispensable role of Tregs in immune tolerance and anticancer activity of the destabilized Tregs (C).
- PDF, 1984-1988, Dept. Biology, MIT, USA
- Ph.D., 1984, Dept. Pharma. Chem., Univ. Calif. San Francisco, USA
- MS, 1979, Dept. Biol. Chem., Univ. Illinois, USA
- BS, 1974, Dept. Pharmacy, Natl. Taiwan Univ.
- Hsiao, H.W., Liu, W.H., Wang, C.J., Lo, Y.H., Wu, Y.H., Jiang, S.T., Lai, M.Z. (2009) Deltex1 is a target of the transcription factor NFAT that promotes T cell anergy. Immunity 31: 72-83.
- Hsieh, W.C., Chuang, Y.T., Chiang, I.H., Hsu, S.C., Miaw, S.C., Lai, M.Z. (2014) Inability to resolve specific infection generates innate immunodeficiency syndrome in Xiap-/- mice. Blood 124: 2847-2857.
- Hsiao, H.W., Hsu, T.S., Liu, W.H., Hsieh, W.C., Chou, T.F., Wu, Y.J., Jiang, S.T., Lai, M.Z. (2015) Deltex1 antagonizes HIF-1α and sustains the stability of regulatory T cells in vivo. Nature Communications 6: 6353. doi: 10.1038/ ncomms7353.
- Chou, T.F., Chuang, Y.T., Hsieh, W.C., Chang, P.Y., Liu, H.Y., Mo, S.T., Hsu, T.S., Miaw, S.C., Chen, R.H., Kimchi, A., Lai, M.Z. (2016) Tumor suppressor deathassociated protein kinase targets cytoplasmic HIF-1α for Th17 suppression. Nature Communications 7: 11904, doi: 10.1038/ ncomms11904.
- Hsieh, W.C., Hsu, T.S., Chang, Y.J., Lai, M.Z.. (2018) IL-6 receptor blockade corrects defects of XIAPdeficient regulatory T cells. Nature Communications 9:463. doi: 10.1038/s41467-018-02862-4.
- Hsu, T.S., Lin, Y.L., Wang, Y.A., Mo, S.T., Chi, P.Y., Lai, A.C.Y., Pan, H.Y., Chang, Y.J., Lai, M.Z. (2020) Hypoxiainducible factor 2α is indispensable for regulatory T cell function. Nature Communications 11: 5005. doi: 10.1038/s41467-020-18731-y.