軸突連結、海馬迴與老化
我們致力於研究神經軸突連結如何形成(axon guidance)。此領域探索所有與大腦神經細胞連結與連結後修飾的所有細節;從早期發育到個體成熟,終其一生,迴路的連結不斷變化。 在成年的哺乳類大腦,海馬迴齒狀迴(hippocampal dentate gyrus)內的下顆粒層區域(subgranular zone)始終保留神經新生(neurogenesis)的能力。 在成年時才新生的神經細胞會被整合進已發育成熟的神經迴路,參與學習與記憶的功能。許多研究顯示,如果海馬迴神經新生功能異常,將造成阿茲海默失智症、憂鬱、癲癇以及老化相關的認知功能退化。
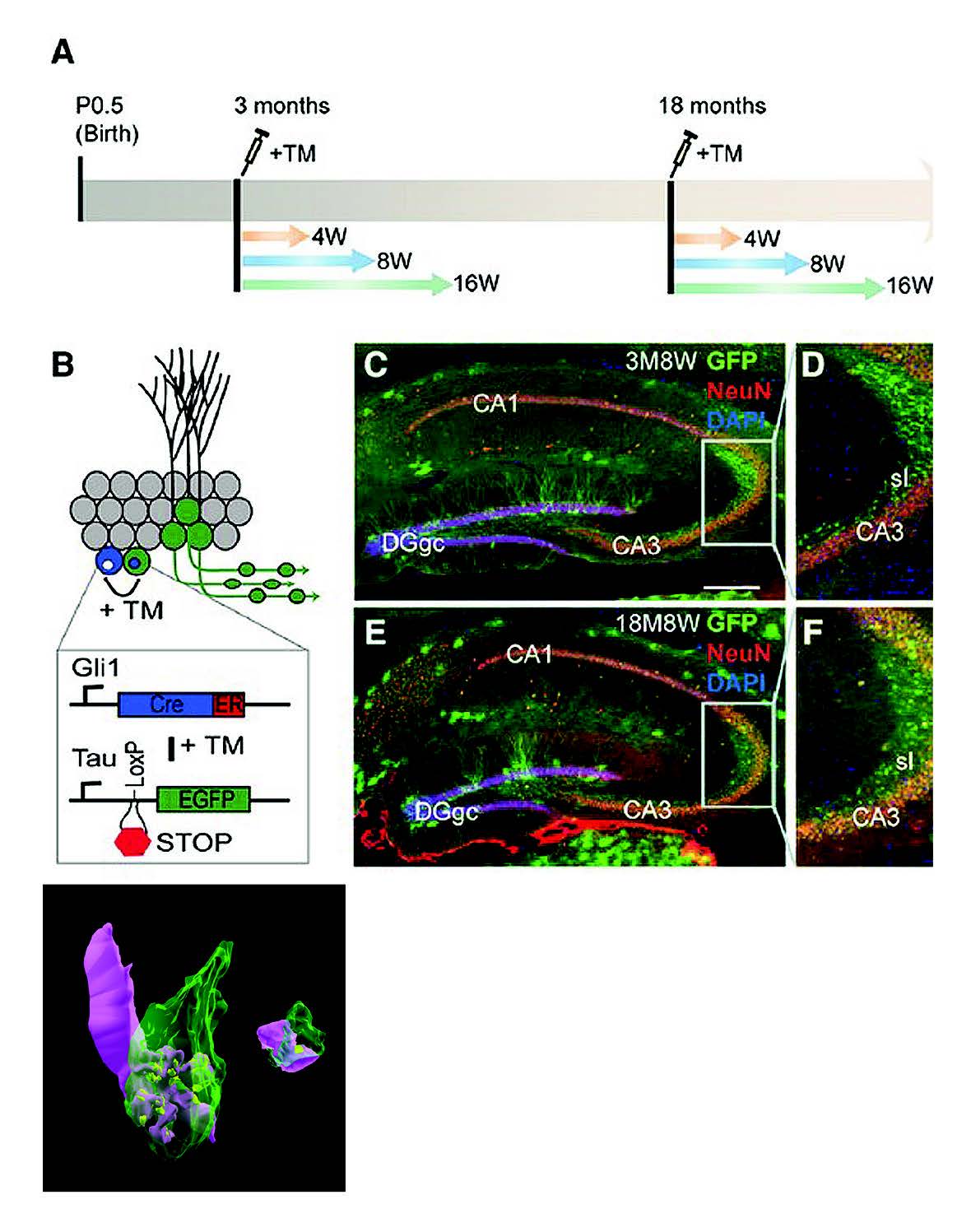
我們研究團隊的目標是釐清在大腦老化過程中,海馬迴神經細胞新生能力退化的原因,以及老化如何改變海馬迴新生神經細胞的軸突連結。 如同已成熟的齒狀迴顆粒細胞(granule cell),成年時才新生的顆粒細胞也會接受來自貫通纖維(perforant pathway)的軸突輸入訊息,再由顆粒細胞軸突聚集成的苔狀纖維(mossy fiber pathway),將訊息送到成熟CA3 錐狀細胞 (pyramidal cell) 的近端樹突。 我們與其他研究團隊都發現,在剛成年的個體,新生顆粒細胞生成的苔狀纖維,其軸突末端(膨體(bouton))要在CA3 錐狀細胞上的巨大棘突(thorny excrescences)形成突觸(synapse)需為期 8週。 成年新生苔狀纖維膨體形成突觸有兩種形式: 1)從無到有的突觸生成(de novo synaptogenesis);2)漸進式地侵入已有的突觸,取代原有的膨體。但其中的發育機制還不明瞭。
我們結合小鼠基因學、共軛焦顯微影像、電子顯微影像及分子陣列層析成像(array tomography-based molecular phenotyping)等技術,勾勒出成年新生的海馬迴顆粒細胞隨年齡的改變過程,並解開老年時新生的顆粒細胞如何產生,以致整合進原有的神經網絡。
- PDF, 1997-2002, University of California San Francisco Stanford University, USA
- Ph.D., 1995, Harvard University, USA
- MD, 1989, College of Medicine, National Taiwan University
- NACADA Region 9 Excellence in Advising Award (2016)
- UC Davis Academic Advising Award (2015)
- Faculty Service Award, Neuroscience Graduate Group, UC Davis (2012)
- Klingenstein Fellowship Award (2004)
- Alfred P. Sloan Research Fellow (2004)
- Whitehall Foundation Grant Award (2003)
- Howard Hughes Medical Institute Physician Postdoctoral Fellowship(1997-2002)
- Pharmacia Biotech & Science Prize for Young Scientists in Molecular Biology, Regional winner from North America (1996)
- Liu, W.-W., Chen, S.-Y., Cheng, C.-H., Cheng, H.-J., Huang, P.-H. (2014) Blm-s, a BH3-only protein enriched in postmitotic immature neurons, is transcriptionally upregulated by p53 during DNA damage. Cell Reports 9: 166-179.
- Failor, S., Chapman, B., Cheng, H.-J. (2015) Retinal waves regulate afferent terminal targeting in the early visual pathway. Proc. Natl. Acad. Sci. USA 112: E2957-E2966.
- Davis, Z. W., Chapman, B., Cheng, H.-J. (2015) Increasing spontaneous retinal activity before eye opening accelerates the development of geniculate receptive fields. J. Neurosci. 35:14612-14623.
- Chen, S.-Y., Ho, C.-T., Liu, W.-W., Lucanic, M., Shih, H.- M., Huang, P.-H., Cheng, H.-J. (2018) Regulation of axon repulsion by MAX-1 SUMOylation and AP-3. Proc. Natl. Acad. Sci. USA 115: E8236-E8245.
- Murray, K. D., Liu, X.-B., King, A. N., Luu, J., Cheng, H.-J. (2020) Age-related changes in synaptic plasticity associated with mossy fiber terminal integration during adult neurogenesis. eNeuro 7(3): ENEURO.0030-20.