Immunotherapy for Metastatic Cancer
Our research interest has been to develop effective immunotherapy for treating metastatic cancer and to investigate the mechanisms underlying the treatment efficacy. We studied autologous natural killer (NK) cell therapy, a clinically used chemotherapy drug cyclophosphamide (CTX) and their combination using a syngeneic orthotopic EO771 breast cancer model that displays chromosomal instability and spontaneous metastasis.
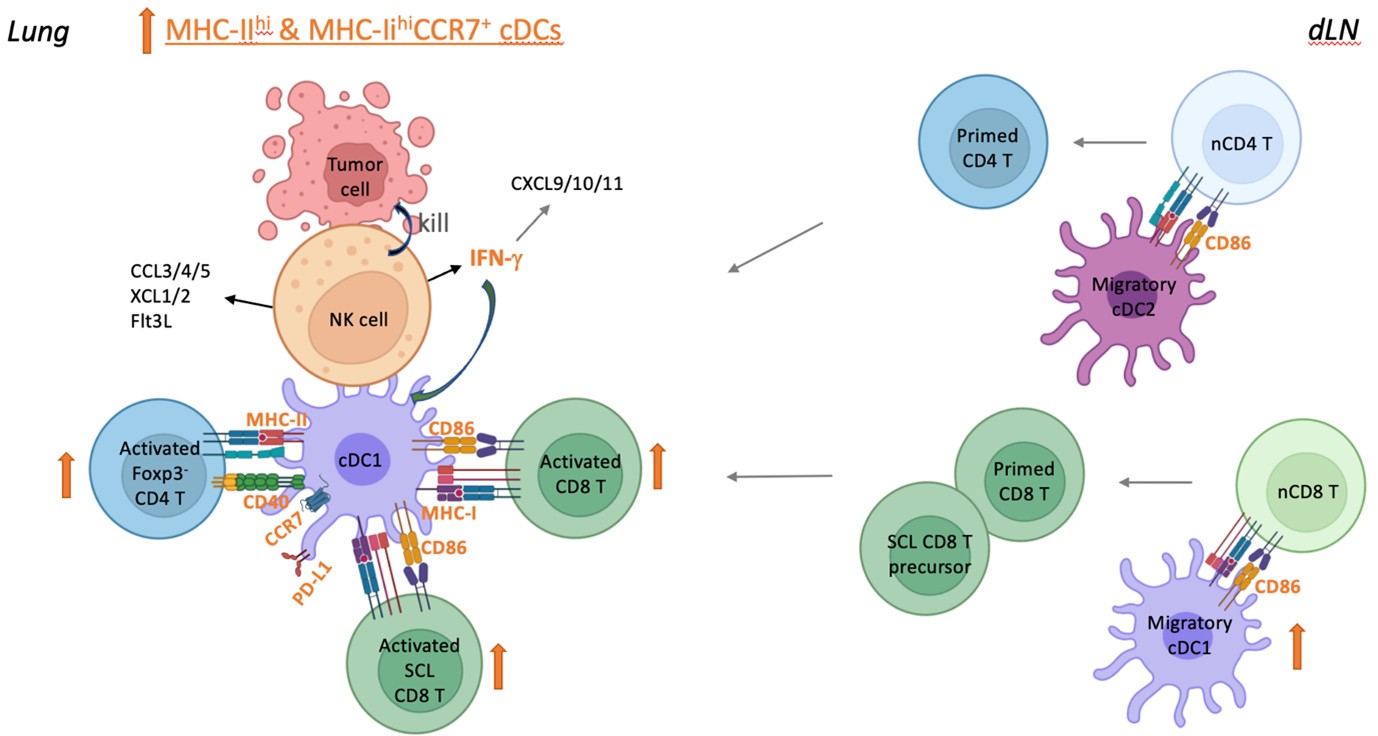
Syngeneic NK cell therapy augments anti-tumor T cell responses through activation of conventional dendritic cells in the metastatic lung
We expanded and conditioned syngeneic NK cells in interleukin 15 (IL-15) and IL-12 ex vivo, and transferred them into mice with lung metastasis after resecting the primary tumor and the draining lymph node (dLN) to mimic cancer recurrence at distal site in patients received surgical treatment. We found that the syngeneic NK cell therapy promotes long-term survival of tumor-resected mice with low metastatic burden and induces protective tumor-specific T cell memory. Furthermore, transfer of NK cells augments activation of type 1 and type 2 conventional dendritic cells (cDC1s and cDC2s), Foxp3-CD4+ T cells and stem cell-like (SCL) CD8+ T cells in metastatic lungs, to which IFN-γ of the transferred NK cells contributes significantly. These results imply direct interactions between the transferred NK cells and endogenous cDCs to enhance T cell activation. We also conducted an investigator-initiated clinical trial of autologous NK cell therapy in six patients with advanced cancer and observed that the NK cell therapy was safe and showed signs of effectiveness. These findings indicate that autologous NK cell therapy is effective in treating established low burden metastases by activating the cDC-T cell axis at metastatic sites.
A model for the augmentation of anti-tumor T cell responses by autologous NK cell therapy in metastatic lung. Autologous NK cells are expanded and conditioned in IL-15 and IL-12 ex vivo. The adoptively transferred NK cells enter the metastatic lung tissue and directly interact with cDCs, which promotes the activation of cDC1 and cDC 2 and the subsequent activation of CD4+ and CD8+ T cells. The increase of activated cDCs in the lung tissue likely leads to an increase of migratory cDCs in the dLN, which results in an increase of primed T cells in dLN that home to the lung tissue. IFN-γ of the transferred NK cells contribute significantly to the activation of cDCs and T cells in metastatic lung.
Effective treatment of advanced breast cancer by CTX requires GAS and STING in host myeloid cells
We found that CTX therapy promotes long-term survival of mice with high burden metastatic EO771 breast cancer. Given that CTX induces DNA damage of tumor cells and increases systemic type I interferon (IFN-I), we investigated the role of host cGAS and STING in the anti-tumor effect of CTX in vivo. We found that the effectiveness of CTX therapy depends on CD8+ T cells and cGAS/STING of bone marrow (BM)-derived cells. Furthermore, STING of cDC1s and LysM+ cells, and IFN-I response of non-cDC1 myeloid cells are essential for CTX efficacy. We also found that cGAS and STING of BM-derived cells positively modulate intratumoral exhausted and SCL CD8+ T cell populations under CTX treatment with the latter only being affected by cGAS. Our study elucidates that the CD8+-T-cell-dependent anti-tumor mechanisms of CTX critically involve the cGAS-STING-IFN-I axis, IFN-I response, and STING-independent cGAS function in host myeloid cells. These findings suggest the deployment of CTX in treating advanced solid tumor to bypass the failure of IFN-I production by tumor cells due to coping with chronic activation of intrinsic cGAS-STING caused by chromosomal instability.
A model for CD8+ T cell-dependent anti-tumor effect of CTX therapy via activation of cGAS and STING in host myeloid cells.The active metabolite of CTX induces the immunogenic death of proliferating tumor cells via dsDNA crosslinking. The dsDNA released from the dead tumor cells effectively targets APCs and triggers the cGAS–STING–IFN-I pathway. The STING of cDC1s and macrophages and the IFN-I response of certain LysM+ or/and CD11c+ non-cDC1 myeloid cells are essential for CTX efficacy. Under CTX treatment conditions, the cGAS and STING of BM-derived cells facilitate a CD8+ T cell response in tumors by suppressing the levels of CD8+ TEX cell population and the Lag-3 and Tim-3 expression by PD-1hiCD8+ T cells, while the cGAS of BM-derived cells sustains the level of CD8+ TSCL cell population independent of STING.
We further found that, in comparison to single therapy, combination of CTX chemotherapy with syngeneic NK cell therapy significantly improves the efficacy as measured by long-term survival in treating high burden metastatic EO771 breast cancer, suggesting the use of this combination immunotherapy for treating advanced cancer.
- PDF, 1991, Dept. Mol. & Cell Biol. UC-Berkeley, USA
- PDF, 1987-1990, Ctr. Cancer Res. M.I.T., USA
- Ph.D., 1986, Dept. Biol. Sci. Illinois State Univ., USA
- BS, 1980, Dept. Nutri. & Food Sci. Fu-Jen Catholic Univ.
- Chu, C.-L., Chen, S.-S., Wu, T.-S., Kuo, S.-C., and Liao, N.-S. (1999) Differential effects of IL-2 and IL-15 on the death and survival of activated TCRγδ+ intestinal intraepithelial lymphocytes. J. Immunol. 162:1896, 10.4049/jimmunol.162.4.1896
- Lai, Y.-G., Gelfanova, V., Kulik, L., Chu, C.-L., Jeng, S.-W., and Liao, N.-S. (1999) IL-15 promotes survival but not effector function differentiation of CD8+ TCRαβ+ intestinal intraepithelial lymphocytes. J. Immunol. 163:5843, DOI: 10.4049/jimmunol.163.11.5843
- Lai, Y.-G., Hou, M.-S., Hsu, Y.-W., Chang, C.-L., Liou, Y.-H., Tsai, M.-H., Lee F., and Liao, N.-S. (2008) IL-15 does not affect IEL development in the thymus but regulates homeostasis of putative precursor and mature CD8aa+ IELs in the intestine. J. Immunol. 180: 3757, DOI: 10.4049/jimmunol.180.6.3757
- Lee, G.A., Wang, S.-W., Liou, Y.-H., Jiang, S.-T., Ko, K.-L. and Liao, N.-S.* (2011) Different NK cell developmental events require different levels of IL-15 trans-presentation. J. Immunol. 187:1212-21, DOI: 10.4049/jimmunol.1100331
- Chang, C.-L., Lai, Y.-G., Hou, M.-S., Huang, P.-L. and Liao, N.-S.* (2011) IL-15R expressed by radiation-resistant cells is necessary and sufficient for thymic iNKT cell survival and functional maturation. J. Immunol. 187:1235-42, doi: 10.4049/jimmunol.1100270
- Lai, Y.G., Hou, M.S., Lo, A., Huang, S.T., Huang, Y.W., Yang-Yen, H.F., Liao, N.S. (2013) IL-15 modulates the balance between Bcl-2 and Bim via Jak3/1- PI3K-Akt-ERK pathway to promote CD8αα+ intestinal intraepithelial lymphocyte survival. Eur. J. Immunol. 43: 2305, DOI: 10.1002/eji.201243026
- Liou, Y.H., Wang, S.W., Chang, C.L., Huang, P.L., Hou, M.S., Lai, Y.G., Lee, G.A., Jiang, S.T., Tsai, C.Y., Liao, N.S. (2014) Adipocyte IL-15 regulates local and systemic NK cell development. J. Immunol. 193: 1747, DOI: 10.4049/jimmunol.1400868.
- Hou, M.S., Huang, S.T., Tsai, M.H., Yen, C.C., Lai, Y.G., Liou, Y.H., Lin, C.K., Liao, N.S. (2015) The interleukin-15 system suppresses T cell-mediated autoimmunity by regulating negative selection and nT17 cell homeostasis in the thymus. J. Autoimmunity 56: 118, doi: 10.1016/j.jaut.2014.11.003.
- Huang, P.L., Hou, M.S., Wang, S.W., Chang, C.L., Liou, Y.H., Liao, N.S. (2015) Skeletal muscle interleukin 15 promotes CD8+ T-cell function and autoimmune myositis. Skeletal Muscle 5: 33, DOI: 10.1186/s13395-015-0058-2
- Lee, G.A., Lai, Y.G., Chen, R.J., Liao, N.S. (2017) Interleukin 15 activates Akt to protect astrocytes from oxygen glucose deprivation-induced cell death. Cytokine 92: 68, doi: 10.1016/j.cyto.2017.01.010.
- Lee, G.A., Lin, T.N., Chen, C.Y., Mau, S.Y., Huang, W.Z., Kao, Y.C., Ma, R.Y., Liao, N.S. (2018) Interleukin 15 blockade protects the brain from cerebral ischemiareperfusion injury. Brain Behav. Immnu. 73: 562, doi: 10.1016/j.bbi.2018.06.021.
- Lee, G.A., Chang, C.M., Wu, Y.C., Ma, R.Y., Chen, C.Y., Hsue, Y.T., Liao, N.S.*, Chang, H.H.* (2021) Chinese herbal medicine SS-1 inhibits T cell activation and abrogates TH responses in Sjögren’s syndrome. J. Formos. Med. Assoc. 120: 651, doi: 10.1016/j.jfma.2020.07.024.
- Huang SW, Lai YG, Liao HT, Chang CL, Ma RY, Chen YH, Liou YH, Wu ZQ, Wu YC, Liu KJ, Huang YT, Yang JL, Dai MS*, and Liao NS* (2025) Syngeneic natural killer cell therapy activates dendritic and T cells in metastatic lungs and effectively treat low-burden metastases. eLife DOI: 10.7554/eLife.99010.3
- Lai YG, Liao HT, Chen YH, Huang SW, Liou YH, Wu ZQ, and Liao NS (2025) cGAS and STING in host myeloid cells are essential for effective cyclophosphamide treatment of advanced breast cancer. Cancers 17:1130, DOI: 10.3390/cancers17071130