Dr. Hsueh-Chi Yen discover the mechanism of protein stability regulated by C-termini
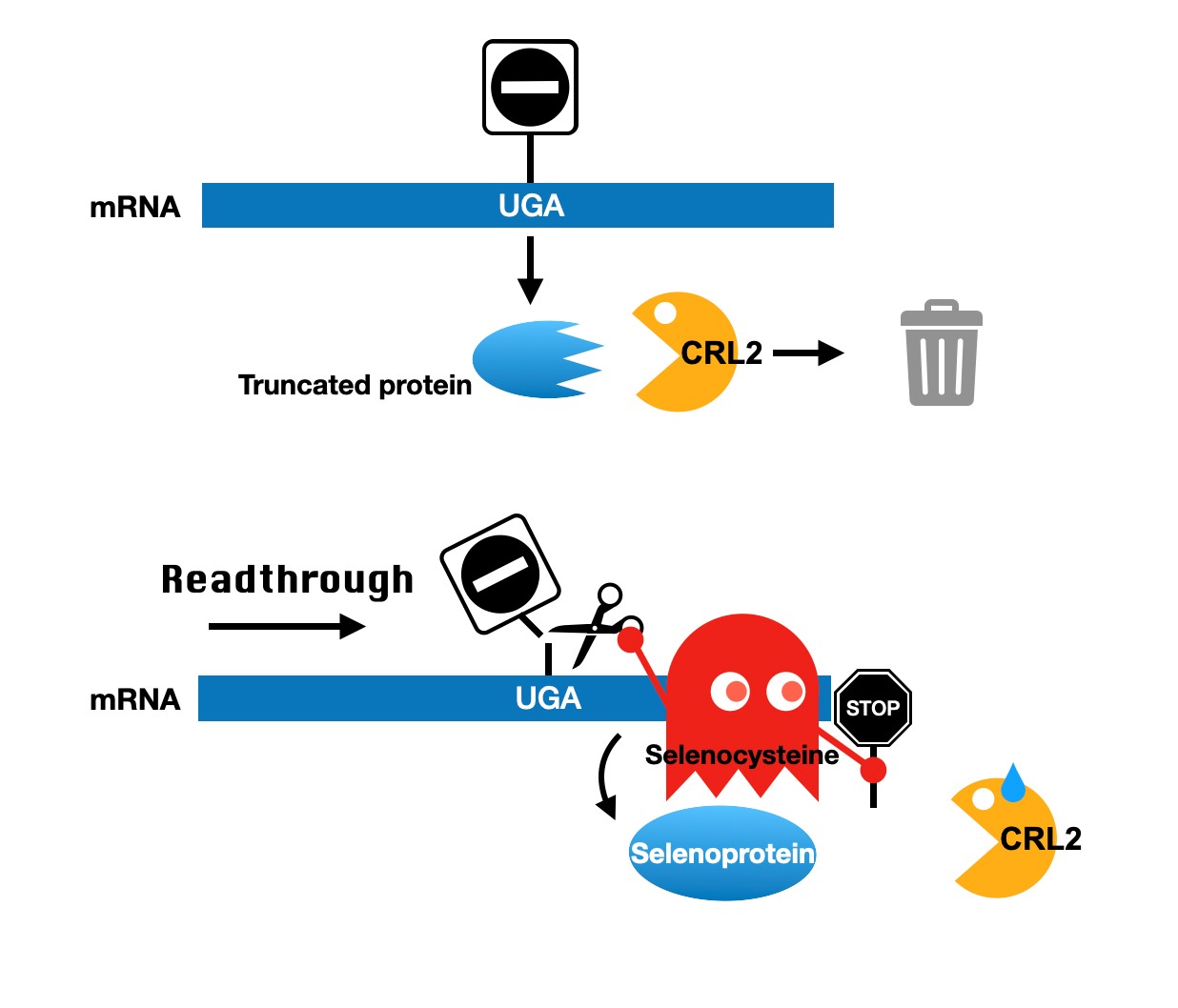
Proteome fidelity is of critical importance in almost all cellular processes, yet it is constantly challenged by a diversity of protein aberrations arising from genetic mutations, erroneous transcription and translation, improper folding, and damage induced by various environmental stresses. Selenocysteine (Sec) is the 21st amino acid in protein synthesis and translated by the UGA codon. However, the UGA codon is recognized as termination signal and translated mRNA into truncated protein in the deficiency of selenium. How can such a side spectrum of defective proteins be captured? Our study addresses these long-standing fundamental questions, which have a broad impact on biology. Our findings reveal that CRL2 ubiquitin ligase-mediated protein quality-control system which specifically eliminates truncated proteins. Moreover, our lab recently discovered that protein C-termini not only play a role in protein quality control but also participate in other regulatory mechanisms. In particular, we demonstrated that C-terminal extensions caused by nonstop mutations can regulate protein stability and substantially affect the function of cellular proteins, especially those involved in cancer and genetic diseases.