Neuronal Morphogenesis And Circuit Formation In Autism Spectrum Disorders
My laboratory has a long-term interest in exploring how genes control neuronal morphology and activity and thereby regulate neural circuit formation and behaviors. We have been focusing on causative genes for neurodevelopmental disorders (ND), especially autism spectrum disorders (ASD) because ASD arises from aberrant neural development, which consequently results in abnormal neural connectivity. We have been using genetically modified mouse models and cultured hippocampal and cortical neurons to investigate how mutations of causative genes for ASD lead to altered neuronal morphology and function and abnormal mouse behaviors. We adopt multiple different experimental systems for our research. First, we apply biochemical and cell biology approaches to investigate the protein-protein interactions and subcellular distributions of target proteins in neurons. Based on results from cultured neurons and data on mutations in patients, we establish mouse genetic models to characterize the functions of those proteins in controlling brain anatomy and mouse behaviors. We then use electrophysiological recording, optogenetic and chemogenetic approaches to investigate how those proteins control neuronal activity and circuits. To validate the molecular mechanisms we identify, we always conduct rescue experiments as a follow-up. Importantly, those rescue experiments often provide useful information on potential therapeutic agents for autism. In the future, we wish to pursue this research direction further to investigate how ASD-linked genes control neuronal morphology and circuits. Given that ASD is male-biased, we will also investigate what factors contribute to the sex bias of ASD, shedding further light on its molecular etiology.
- PDF, 1996-2000, HHMI & Massachusetts General Hospital & Harvard Medical School, USA
- Ph.D., 1995, Graduate Institute of Microbiology & Immunology, National Yang-Ming University
- BS, 1987, Department of Biology, National Taiwan Normal University
- 1986, Tung Di-Gung Award, Ministry of Education (for the student who ranks first at the School of Science, National Taiwan Normal University, Taipei)
- 1991, Scholarship of Chinese Society for Microbiology and Immunology for outstanding graduate student in Immunology.
- 1995, Wang Ming-Ning Award for Medical Ph.D. Thesis.
- 1999, Young Investigator Award, National Neurofibromatosis Foundation, New York, New York.
- 2001, Li Foundation Heritage Prize for Scholarly Development in recognition of research contribution in the field of molecular neurobiology.
- 2005, Academia Sinica Research Award for Young Investigators (年輕學者研究著作獎)
- 2005-2010, 2010-2015, 2015-2020, 2023-2028, Frontier Science Research Grant, National Science and Technology Council or Ministry of Science and Technology (科技部或國科會尖端計畫四次)
- 2008, The 4rd TienTe Lee Award–Young Scientists, TienTe Lee Biomedical Foundation (李天德獎)
- 2016, Explorer Award, Simons Foundation Autism Research Initiative, USA.
- 2017-2022, 2022-2026, Academia Sinica Investigator Award (二次)
- 2019, The 15th TienTe Lee Award–Outstanding, TienTe Lee Biomedical Foundation (李天德獎)
- 2007-2010, 2013-2016, 2016-2019, Distinguished Scholars Research Award, National Science and Technology Council, Ministry of Science and Technology or National Science Council(科技部或國科會傑出研究獎三次)
- 2021 65th Academic Award of Ministry of Education (教育部學術獎)
- 2022, Merit NSTC Research Fellow, NSTC (國科會傑出特約研究員獎)
- Hsu, T.-T., Chen, C.-P.#, Lin, M.-H.#, Hong, T.-E.#, Huang, T.-N.#, Wang, C-Y.*, and Hsueh, Y.-P.* (2026) Shared and divergent alteration of whole-brain connectivity and sensory deficits in multiple autism mouse models. Molecular Psychiatry (Early access in November 2025, https://doi.org/10.1038/s41380-025-03340-2)(co-corresponding authors; # co-second authors) (an invited blog in “Behind the paper” of Nature Research Communities)
- Huang, T.-N.#, Lin, M.-H. #, Hsu, T.-T., Yu, C.-H., Hsueh, Y.-P.* (2025) Low-dose mixtures of dietary nutrients ameliorate behavioral deficits in multiple mouse models of autism. PLOS Biology 23:e3003231 (Selected by PLOS Biology for press release)
- Hsueh, Y.-P.* (2025) Signaling in Autism: Relevance to Nutrients and Sex. Current Opinion in Neurobiology 90:102962. (Invited review)
- Hsu, T.-T., Huang, T.-N., Wang, C.-Y., and Hsueh, Y.-P.* (2024) Deep brain stimulation of the Tbr1-deficient mouse model of autism spectrum disorder at the basolateral amygdala alters amygdalar connectivity, whole-brain synchronization, and social behaviors. PLOS Biology 22(7): e3002646.
- Chao, Y.-W., Lee, Y.-L., Tseng, C.-S., Wang, L. U.-H., Hsia, K.-C., Chen, H., Fustin, J.-M., Azeem, S., Chang, T.-T., Chen, C.-Y., Kung, F.-C., Hsueh, Y.-P.*, Huang, Y.-H.*, Chao, H.-W.* (2024) Improved CaP-nanoparticles for Nucleic Acid and Protein Delivery to Neural Primary Cultures and Stem Cells. ACS Nano 18:4822-4839.
- Hu, H.-T., Lin, Y.-J., Wang, U.-T.T., Lee, S.-P., Liou, Y.-H., Chen, B.-C., and Hsueh, Y.-P.* (2023) Autism-related KLHL17 and SYNPO act in concert to control activity-dependent dendritic spine enlargement and spine apparatus. PLOS Biology 21: e3002274.
- Yen, T.-L., Lin, M.-H., Lu, M.-H., Hsu, T.-T., Huang, T.-N. Shih, P.-Y., Ellegood, J., Lerch, J. and Hsueh, Y.-P.* (2023) Sex bias in social deficits, neural circuits and nutrient demand in Cttnbp2 autism models. Brain 146:2612-2626.
- Shih, P.-Y.#, Fang, Y.-L.#, Shankar, S., Lee, S.-P., Chen, H., Wang, T.-F., Hsia, K.-C.*, Hsueh, Y.-P.* (2022) Phase separation and zinc-induced transition modulate synaptic distribution and association of autism-linked CTTNBP2 and SHANK3. Nature Communications 13:2664. (Featured by Nature Communications Editor’s Highlights webpage, showcases research in “Protein Liquid-Liquid Phase Separation in diseases”, and an invited blog in “Behind the paper” of Nature Portfolio Communities.)
- Shih, Y.-T.#, Huang, T.-N.#, Hu, H.-T.#, Yen, T.-L., and Hsueh, Y.-P.* (2020) Vcp overexpression and leucine supplementation increase protein synthesis and improve fear memory and social interaction of Nf1 mutant mice. Cell Reports 31:107835.
- Shih, P.-Y., Hsieh, B.-Y., Lin, M.-H., Huang, T.-N., Tsai, C.-Y., Pong, W.-L., Lee, S.-P., and Hsueh., Y.-P.* (2020) CTTNBP2 controls synaptic expression of zinc-related autism-associated proteins and regulates synapse formation and autism-like behaviors. Cell Reports 31: 107700.
- Huang, T.-N.#, Hsu, T.-T.#, Hu, H.-T., Chuang, H.-C., Sun, T.-F., Tao, M.-H., Lin, J.Y., and Hsueh, Y.-P.* (2019) Interhemispheric connectivity potentiates the basolateral amygdalae and regulates social interaction and memory. Cell Reports 29:34-48.
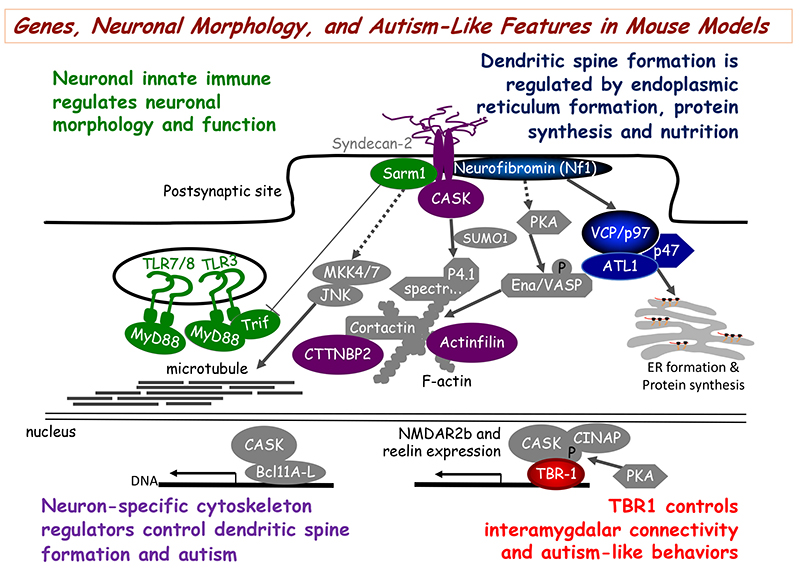
- Hung, Y.-F., Chen, C.-Y., Shih, Y.-C., Liu, H.-Y., Huang, C.-M., and Hsueh, Y.-P.* (Aug, 2018) Endosomal TLR3, TLR7, and TLR8 control neuronal morphology through different transcriptional programs. Journal of Cell Biology 217:2727-2742.
- Chen, C.-Y., Liu, H.-Y., and Hsueh, Y.-P.* (2017) TLR3 downregulates expression of schizophrenia gene Disc1 via MYD88 to control neuronal morphology. EMBO Reports 18:169-183.
- Shih, Y.-T. and Hsueh, Y.-P.* (2016, March) VCP and ATL1 regulate endoplasmic reticulum and control protein synthesis for dendritic spine formation. Nature Communications 7:11020.
- Lee, E.-J., Lee, H., Huang, T.-N., Chung, C., Shin, W., Kim, K., Koh, J.-Y., Hsueh, Y.-P.* and Kim, E.* (2015) Trans-synaptic Zinc mobilization improves social interaction in two mouse models of autism through NMDAR activation. Nature Communications 6:7168.
- Huang, T.-N., Chuang, H.-C., Chou, W.-H., Chen, C.-Y., Wang, H.-F., Chou, S.-J., and Hsueh, Y.-P.* (2014) Tbr1 haploinsufficiency impairs amygdalar axonal projections and results in cognitive abnormality. Nature Neuroscience 17:240-247.
- #Wang, H.-F., #Shih, Y.-T., #Chen, C.-Y., Chao, H.-W., Lee, M.-J.*, and Hsueh, Y.-P.* (2011) Valosin-containing protein and neurofibromin interact to regulate dendritic spine density. Journal of Clinical Investigation 121:4820-4837. Highlighted by a commentary in the same issue of JCI
- Chen, C.-Y., Lin, C.-W., Chang, C.-Y., Jiang, S.-T., and Hsueh, Y.-P.* (2011) Sarm1, a negative regulator of innate immunity, interacts with syndecan-2 and regulates neuronal morphology. Journal of Cell Biology 193:769-784.
- Hsueh, Y.-P.* (2009) Calcium/Calmodulin-Dependent Serine Protein Kinase and Mental Retardation. Annals of Neurology 66:438-443.
- Chao, H.-W., Hong, C.-J., Huang, T.-N., Lin, Y.-L., and Hsueh, Y.-P.* (2008) SUMOylation of the MAGUK protein CASK regulates dendritic spinogenesis. Journal of Cell Biology 182:141-155. #Highlighted by Faculty of 1000 Biology
- Lin, Y.-L., Lei, Y.-T., Hong, C.-J., and Hsueh, Y.-P.* (2007) Syndecan-2 induces filopodia and dendritic spine formation via the neurofibromin-PKA-Ena/VASP pathway. Journal of Cell Biology 177:829-841.
- Wang, G.-S., Hong, C.-J., Yen, T.-Y., Huang, H.-Y., Ou, Y., Huang, T.-N., Jung, W.-G., Kuo, T.-Y., Sheng, M., Wang, T.-F., and Hsueh, Y.-P.* (2004) Transcriptional Modification by a CASK interacting nucleosome assembly protein. Neuron 42:113-128.
- Hsueh, Y.-P., Wang, T.-F., Yang, F.-C., Sheng M.* (2000) Nuclear translocation and transcription regulation by the membrane-associated guanylate kinase CASK/LIN-2. Nature 404:298-302.
- Hsueh, Y.-P., Yang, F.-C., Kharazia, V., Naisbitt, S., Cohen, J. M., Weinberg, R. J., and Sheng, M.* (1998). Direct interaction of CASK/LIN-2 and syndecan heparan sulfate proteoglycan and their overlapping distribution in neuronal synapses. Journal of Cell Biology 142:139-151.
- Hsueh, Y.-P., Kim, E., and Sheng, M.* (1997). Disulfide-linked head-to-head multimerization in the mechanism of ion channel clustering by PSD-95. Neuron 18: 803-814.
- Hsueh, Y.-P. and Lai, M.-Z.* (1995). JNK but not MAP kinase is sensitive to cAMP inhibition in T Lymphocytes. Journal of Biological Chemistry 270: 18094-18098.

