An ATP-independent role for Prp16 in promoting aberrant splicing
Dr. Cheng, Soo-Chen - October, 2023
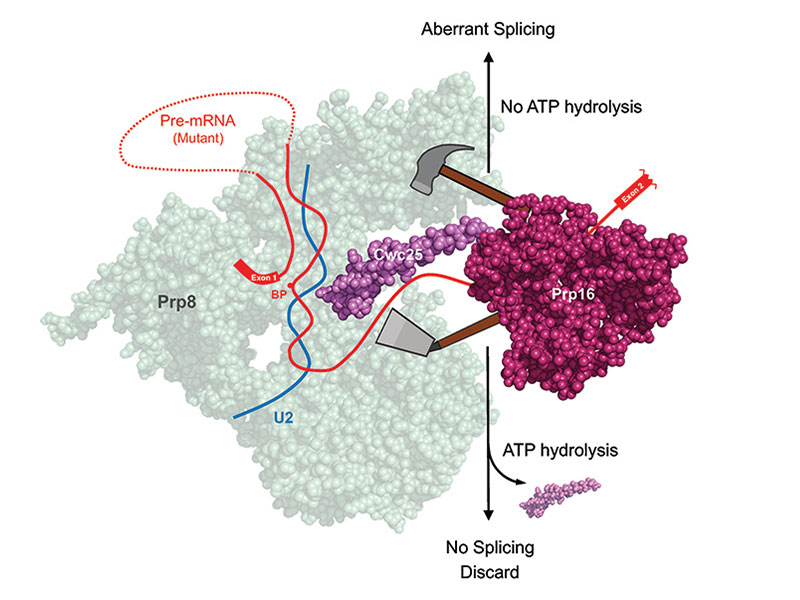
The DExD/H-box RNA helicase Prp16 plays a pivotal role in the splicing process. Following the branching reaction, it utilizes its ATP-dependent functions to facilitate exon ligation by displacing step-one factors, Cwc25 and Yju2, from the spliceosome’s catalytic center, thus enabling the positioning of the 3’ splice site to the catalytic center. Additionally, Prp16 is well known for its role in proofreading the 5’ splice site and the branch site of pre-mRNA, a function that requires ATP. Our research has unveiled a paradoxical role for Prp16 in splicing of pre-mRNA with mutations at splice sites, resulting in a slower splicing reaction. In such cases, Prp16 exhibits an ATP-independent activity by stabilizing the binding of Cwc25, thereby enhancing the branching reaction and leading to aberrant selection of splice sites. This seemly contradictory function of Prp16 raises questions about its role in proofreading splice sites. Nevertheless, it underscores Prp16’s consistent role in propelling the splicing pathway forward. In normal pre-mRNA, Prp16 destabilizes Cwc25 to facilitate exon ligation, whereas for mutant pre-mRNA, it stabilizes Cwc25 to promotes the branching reaction. These discoveries change the current view of splicing fidelity control, leading to the paper’s recognition as Breakthrough Article by NAR.
核醣核酸解旋酶Prp16是剪接反應的一個重要參與因子。在第一步的剪接催化反應後,Prp16會藉由水解ATP將參與第一步反應的核心因子Cwc25和Yuj2從反應中心移除,讓3’剪接位得以進入反應中心以進行第二步反應。Prp16另一個公認的角色是可檢視剪接位的序列,確保剪接反應的準確度,而這個功能也需要ATP。我們的研究發現了Prp16一個不需ATP的新功能。當剪接位有鹼基突變導致剪接反應變慢時,Prp16可藉由將Cwc25穩定在剪接體上來促進第一步反應,但卻會造成錯誤的剪接位選擇。這個研究結果顛覆了過去對於Prp16在剪接反應中扮演校正功能的認知,但卻也確認Prp16對於具正常的或突變的剪接位的前訊息核醣核酸在剪接反應中都扮演推動反應向前的角色:在正常情況下,它利用水解ATP來移除Cwc25以促進第二步反應;而在有突變的狀況下,經由穩定Cwc25來促進第一步反應。這個發現改變了大家對Prp16的角色以及剪接校正的觀點,論文被NAR期刊選為研究的重大突破。