The motor neuron m6A repertoire governs neuronal homeostasis and FTO inhibition mitigates ALS symptom manifestation
Dr. Chen, Jun-An - April, 2025
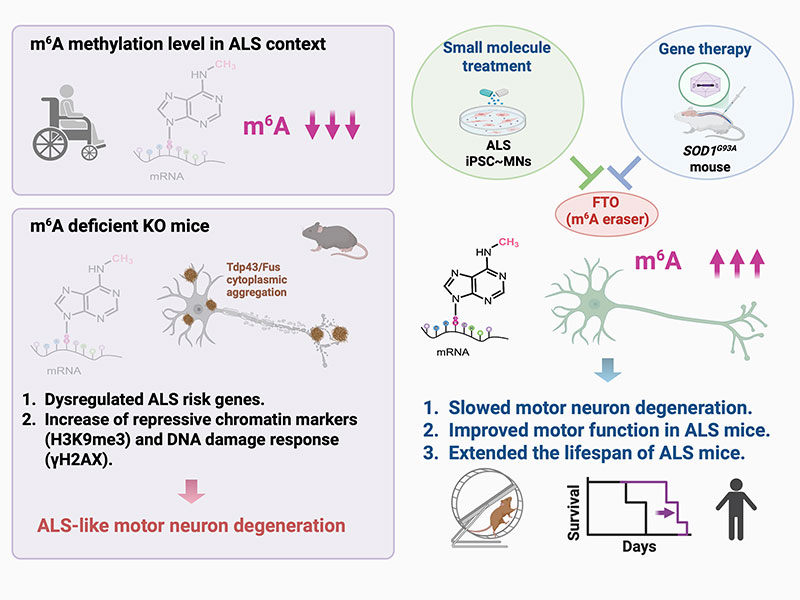
Amyotrophic lateral sclerosis (ALS) is a swiftly progressive and fatal neurodegenerative ailment marked by the degenerative motor neurons (MNs). Why MNs are specifically susceptible in predominantly sporadic cases remains enigmatic. Here, we demonstrated N6-methyladenosine (m6A), an RNA modification catalyzed by the METTL3/METTL14 methyltransferase complex, as a pivotal contributor to ALS pathogenesis. By conditional knockout Mettl14 in murine MNs, we recapitulate almost the full spectrum of ALS disease characteristics. Mechanistically, pervasive m6A hypomethylation triggers dysregulated expression of high-risk genes associated with ALS and an unforeseen reduction of chromatin accessibility in MNs. Additionally, we observed diminished m6A levels in induced pluripotent stem cell derived MNs (iPSC~MNs) from familial and sporadic ALS patients. Restoring m6A equilibrium via a small molecule or gene therapy significantly preserves MNs from degeneration and mitigates motor impairments in ALS iPSC~MNs and murine models. Our study presents a substantial stride towards identifying pioneering efficacious ALS therapies via RNA modifications.
Breakthrough in ALS Research: Homeostasis m6A RNA Modification Opens New Opportunities for ALS Treatment and Biomarkers
The research team led by Dr. Jun-An Chen’s group at the Institute of Molecular Biology, Academia Sinica, has achieved a significant breakthrough in studying Amyotrophic Lateral Sclerosis (ALS) disease. The team discovered that m6A methylation enzymes (Mettl3 and Mettl14) are significantly reduced in human ALS-induced pluripotent stem cell-differentiated motor neurons (iPSC~MNs). This chemical tag acts like a switch on RNA molecules, controlling the accurate transmission of genetic information. When this homeostasis is disrupted, motor neurons age and die. Further experiments demonstrated that specifically knocking out Mettl14 in mouse motor neurons or reducing m6A methylation levels in iPSC~MNs induces neurodegenerative symptoms resembling ALS. By integrating Oxford Nanopore third-generation direct RNA sequencing and 10x Genomics single-nuclei multiomic sequencing, the team not only identified ALS high-risk factors associated with m6A methylation but also found that chromatin remodeling and abnormal histone modifications, triggered by hypomethylation of m6A may be key pathogenic mechanisms in ALS. More excitingly, Dr. Chen’s team further validated that restoring the m6A methylation levels through small-molecule drugs or intrathecal injection delivery in the ALS mouse spinal cord. Both methods significantly increased m6A levels, effectively slowed motor neuron degeneration, improved motor function in animals, and extended the lifespan of ALS mice. This research, published in Nature Communications, provides a new direction for future ALS drug development.
ALS is a progressive neurodegenerative disease with mostly unknown causes and no current cure. While the 2014 global "Ice Bucket Challenge" raised public awareness, it did not lead to fundamental treatments. Now, by regulating the chemical modification of m6A on RNA, Chen’s team has opened new opportunities for treatment and early diagnosis. This strategy demonstrated significant therapeutic effects in a safe and effective m6A-modulating drug, paving a new path for ALS drug development and offering substantial contributions in the ALS field.
The first author of this paper is Dr. Ya-Ping Yen, a postdoctoral researcher at the Institute of Molecular Biology, Academia Sinica. Co-authors include Ting-Hsiang Lung, a master’s student from National Central University, and several research assistants. The research was funded by Academia Sinica, the National Science and Technology Council, the National Health Research Institutes, and the National Biotechnology Research Park.
The study “The Motor Neuron m6A Repertoire Governs Neuronal Homeostasis and FTO Inhibition Mitigates ALS Symptom Manifestation” has been published in Nature Communications.