An interbacterial cysteine protease toxin inhibits cell growth by targeting type II DNA topoisomerases GyrB and ParE
Dr. Ting, See-Yeun - June, 2025
Bacteria deploy a diverse arsenal of toxic effectors to antagonize competitors, profoundly influencing the composition of microbial communities. Previous studies have identified an interbacterial toxin predicted to exhibit proteolytic activity that is broadly distributed among Gram-negative bacteria. However, the precise mechanism of intoxication remains unresolved. Here, we demonstrate that one such protease toxin from Escherichia coli, Cpe1, disrupts DNA replication and chromosome segregation by cleaving conserved sequences within the ATPase domain of type II DNA topoisomerases GyrB and ParE. This cleavage effectively inhibits topoisomerase-mediated relaxation of supercoiled DNA, resulting in impaired bacterial growth. Cpe1 belongs to the papain-like cysteine protease family and is associated with toxin delivery pathways, including the type VI secretion system and contact-dependent growth inhibition. The structure of Cpe1 in complex with its immunity protein reveals a neutralization mechanism involving competitive substrate binding rather than active site occlusion, distinguishing it from previously characterized effector-immunity pairs. Our findings unveil a unique mode of interbacterial intoxication and provide insights into how bacteria protect themselves from self-poisoning by protease toxins.
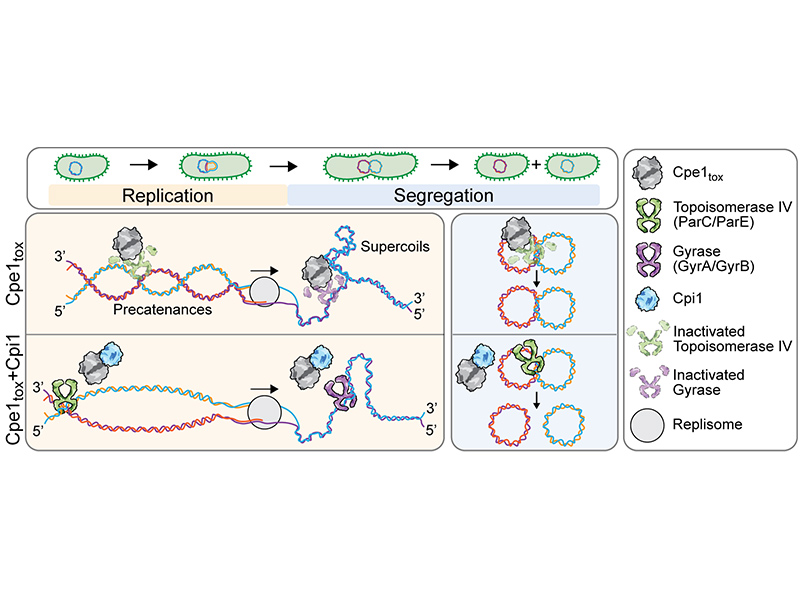
細菌透過多種抗菌毒素對抗競爭對手,進而影響微生物群落的組成。先前的研究已鑑定出一類廣泛存在於革蘭氏陰性菌中的蛋白水解酶型抗菌毒素。然而,其抑制細菌生長的分子機制至今仍不明。中央研究院分子生物研究所陳詩允實驗室的最新研究發現,這類蛋白水解酶毒素(命名為 Cpe1)可特異性水解第二型 DNA 拓撲異構酶,GyrB 和 ParE,從而破壞 DNA 複製過程並干擾細胞分裂時染色體的正常分離。此一水解作用有效抑制了 DNA 拓撲異構酶對超螺旋 DNA 的鬆弛功能,進一步抑制細菌的生長。Cpe1 為木瓜蛋白水解酶半胱氨酸蛋白水解酶家族的成員,能透過第六型分泌系統或接觸依賴性生長抑制系統注入至競爭菌株中。Cpe1 與其對應免疫蛋白的複合物結構揭示了一種新穎的毒素中和機制,該機制並非透過封閉活性位點,而是經由競爭性底物結合來阻斷毒素活性。此研究不只揭示了一種獨特的細菌毒素作用模式,還為細菌如何避免蛋白水解酶毒素造成自我毒害提供了新的分子見解。
本研究於 2025 年 5 月 27 日發表於《PLOS生物學》(PLOS Biology)期刊,領導這項研究的學者為本院國際研究生分子與細胞學程博士候選人宋品儀,並與本院分子生物研究所夏國強研究員及植物與微生物所顧銓副研究員合作。研究經費由本院前瞻計畫及國家科學及技術委員會「2030 跨世代優秀年輕學者計畫」支應。