Shared and divergent alteration of whole-brain connectivity and sensory deficits in multiple autism mouse models
Dr. Hsueh, Yi-Ping - November, 2025
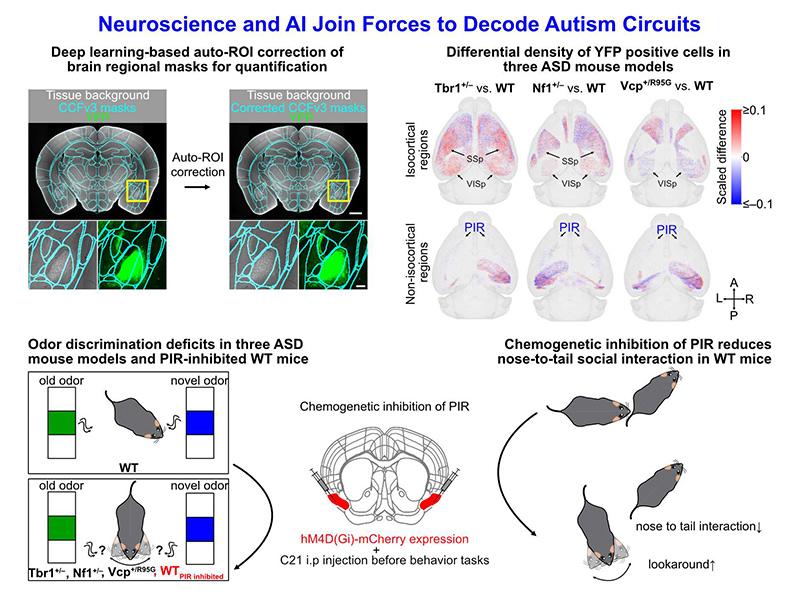
Autism spectrum disorder (ASD) is a heterogeneous developmental disconnection syndrome. Identifying circuit deficits is crucial for understanding ASD etiology, yet the involvement of multiple brain regions and genetic variations complicates this analysis. Here, using an AI-powered mapping platform, BM-auto (Brain Mapping with Auto-ROI correction), to analyze a Thy1-YFP reporter, we show that different ASD-associated mutations cause distinct circuit abnormalities but share common deficits in the piriform cortex, a region regulating olfactory discrimination and social behavior patterns. We analyzed the whole-brain distribution of the Thy1-YFP reporter in three ASD mouse models (Tbr1+/–, Nf1+/–, and Vcp+/R95G). YFP signals revealed altered axonal projections and structural connectivity. We also found that Thy1-YFP+ cell numbers varied across brain regions, revealing deficits in the differentiation or maintenance of projection neurons. While each mutation caused unique connectivity alterations, sensory regions—including the visual, somatosensory, and piriform cortices—were recurrently affected. However, effects on the visual and somatosensory cortices varied between models. The piriform cortex was the only region consistently impaired, showing reduced YFP signals and fewer Thy1-YFP+ neurons across all three models. Furthermore, all three mutants exhibited common olfactory discrimination impairments. Manipulating piriform cortex activity altered social behavior patterns, highlighting its role in ASD-linked circuit dysfunction. These findings underscore the vulnerability of sensory regions—especially the piriform cortex—to ASD-related mutations, strengthening the notion that altered sensory experiences are common in ASD.