cGAS and STING in host myeloid cells are essential for effective cyclophosphamide treatment of advanced breast cancer
Dr. Liao, Nan-Shih - March, 2025
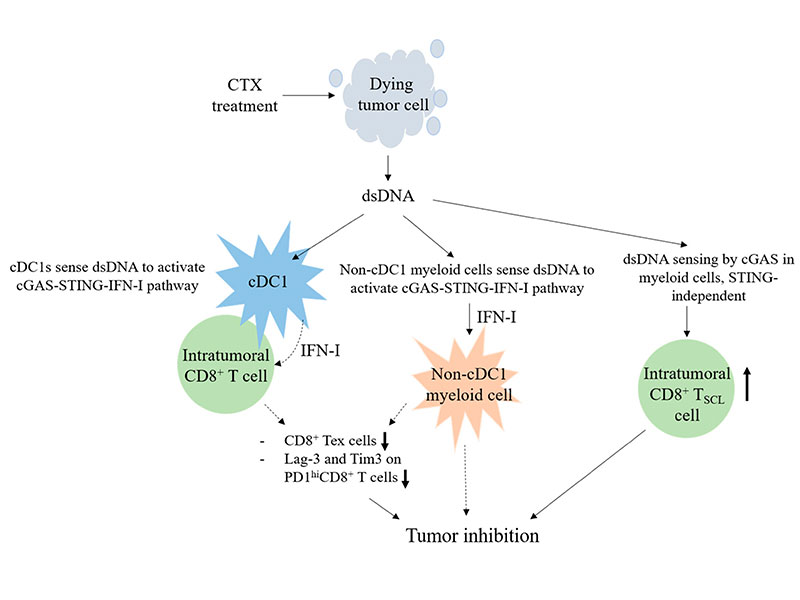
Cyclophosphamide (CTX) treatment in vivo kills proliferating tumor cells by DNA crosslinking, however, suppression of tumor growth by CTX in several murine models requires CD8+ T cells. Given that CTX induces DNA damage and type I interferon (IFN-I), we investigated the role of host cGAS and STING in the anti-tumor effect of CTX in vivo. Using a metastasized EO771 breast cancer model with chromosomal instability, we found that CTX therapy induces long-term survival of the mice with this outcome being dependent on CD8+ T cells and cGAS/STING of bone marrow (BM)-derived cells. Furthermore, STING of type 1 conventional dendritic cells (cDC1s) and LysM+ cells, and IFN-I response of non-cDC1 myeloid cells are essential for CTX efficacy. We also found that cGAS and STING of BM-derived cells positively modulate intratumoral exhausted and stem-cell-like CD8+ T cell populations under CTX treatment with the latter only being affected by cGAS. Our study elucidates that the CD8+-T-cell-dependent anti-tumor mechanisms of CTX critically involve the cGAS-STING-IFN-I axis, IFN-I response, and STING-independent cGAS function in host myeloid cells. These findings suggest the deployment of CTX in treating advanced solid tumor to bypass the often failed IFN-I production by tumor cells due to coping with chronic activation of intrinsic cGAS-STING caused by chromosomal instability.